What’s Inside a Lithium-Ion Battery?
Lithium batteries are the best form of energy storage available today for use on electric boats.
Most boaters who are interested in electric boats don’t know enough about lithium-ion batteries. But we can understand why as it is a confusing subject and making it harder is that there is a lot of misinformation said by everyone from ‘experts’ to bloggers. Our hope is that this series of blogs about lithium batteries helps boaters have a better understanding of them.
This is the first in a six-part series on lithium batteries:
-
-
-
-
- What’s Inside a Lithium-Ion battery?
-
-
-
- How is a Lithium-Ion battery different than a Lead-Acid battery?
- Which is a better Lithium-Ion battery, NMC or LFP?
- Aren’t all Battery Management Systems (BMS) the same?
- What is the future of lithium batteries?
- Beyond Lithium Ion - what’s the future of energy storage and renewable energy generation?
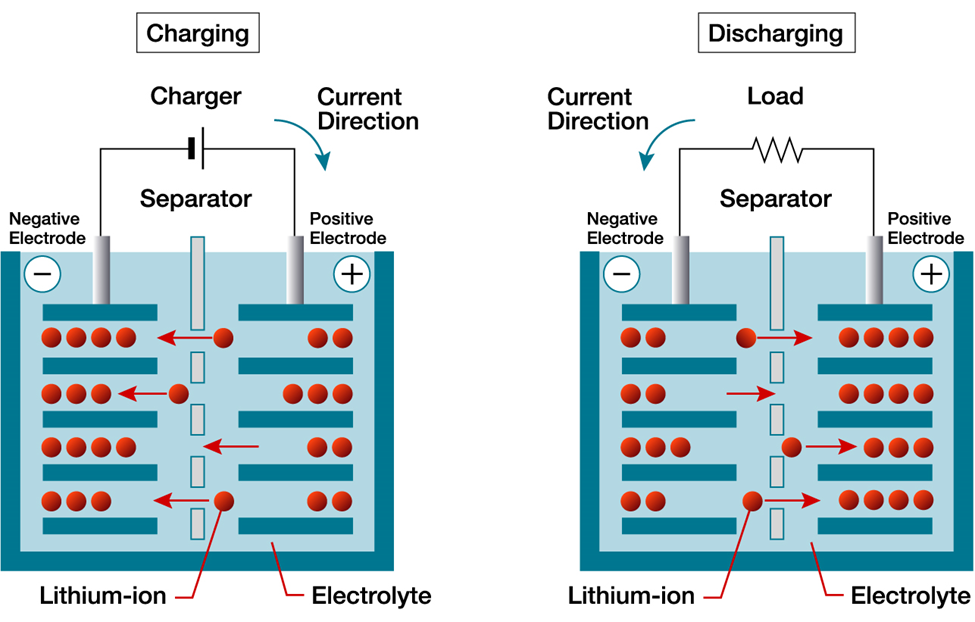
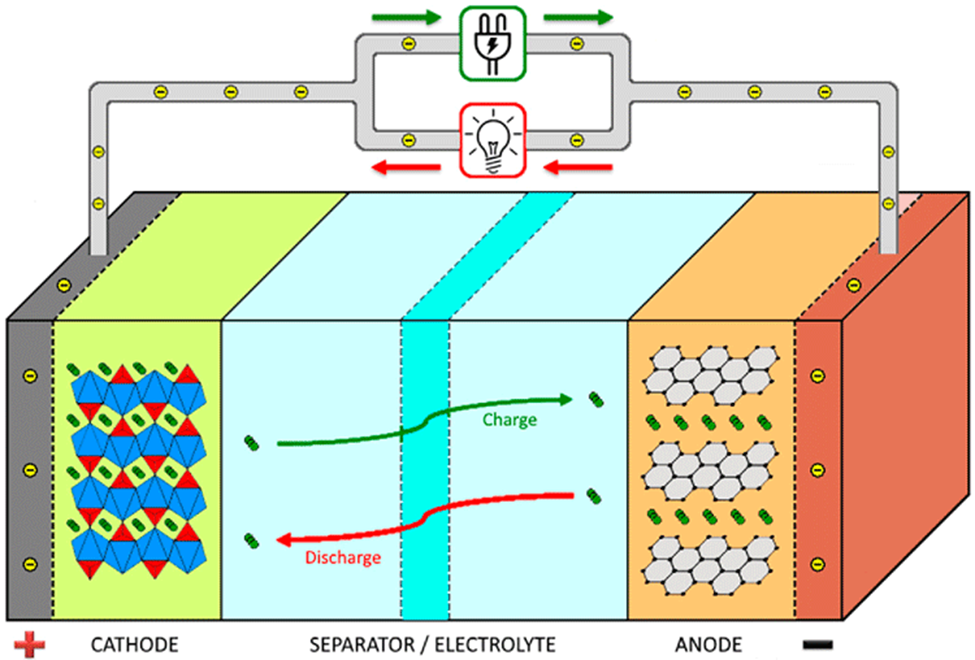
Lithium Ion Battery Explained: First, let’s take a look at what’s in a lithium ion battery. A lithium-ion battery is comprised of an anode, cathode, separator, electrolyte, and two current collectors (positive and negative) attached to terminals that extrude from the battery. The following components are common to both NMC and LFP batteries:
- Electrolyte -a lithium salt, most commonly LiPF6
- Separator -a polymer, most commonly, polyolefin, which is produced from olefin by polymerizing olefin ethylene
- Anode –What is commonly called the negative side of the battery (more on that in just a bit). It is typically made of graphite in lithium-ion batteries.
- Current collectors – Conduct electrons from inside the battery from electrochemical processes to the external terminal. Copper Foil is commonly used for the negative current collector and aluminum for the positive current collector
- Terminals – the external attachment point of a battery that is labeled positive and negative. For marine lithium-ion batteries, these are available in lead, epoxy coated lead, and brass with plated fasteners
- Cathode– there is a reason we put this apart from the Anode in the list. All the components above are the same for different chemistry types of lithium-Ion batteries. It is the cathode material that differs and gives them their respective names. The NMC cathode is made out of Nickle, Manganese and Cobalt (chemical equation: LiMn2O4). The LFP cathode, is made of Lithium Iron Phosphate and Oxygen (chemical equation: LiFePO4)
A lithium-ion battery functions based on creating stored energy potential through charging and releasing that energy potential when discharging. To achieve this, the electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. The movement of the lithium ions creates free electrons in the anode which creates a charge at the positive current collector. The electrical current then flows from the current collector through a device being powered (cell phone, electric motor, etc.) to the negative current collector. The separator blocks the flow of electrons inside the battery in order to create the electron gradient in the circuit. When the battery is charged, the flow of electrical current is reversed and it is the movement of lithium ions back over to the anode from the cathode that creates the energy potential of stored electrons.
We realize that’s a lot of detailed information and probably our most boring blog post in our company’s history. But, it sets the state for the next blog posts in this series on Lithium-Ion batteries. Take a look back at the image and the terms and then continue on to the next blog in the series that looks at the differences between Lithium-Ion and Lead-Acid batteries.







Leave Comment